The MS Studies
MS clinical research studies across the US are actively enrolling participants. You may be eligible for an MS study if you:
- Are 18 to 55 years of age
- Are diagnosed with relapsing-remitting MS
Additional eligibility criteria apply.
MS clinical research studies across the US are actively enrolling participants. You may be eligible for an MS study if you:
Additional eligibility criteria apply.
SOSTOS is a clinical research study that is studying the effects of switching to ofatumumab (study drug) compared to continuing on their current MS treatment for patients who have not had a relapse within the past 12 months. The study will also look at neurofilament light chain (NfL) which is a biomarker that may indicate disease activity.
If you qualify and agree to join SOSTOS, you will be in the study for up to 22 months and asked to come in for office visits about 12 times.
Some of the enrolled study patients will be randomly selected to wear a study watch to collect information on physical activity, sleep, and vitals.
You may be eligible to participate if you:
Patients with RRMS could have ongoing nervous system damage despite no obvious signs or worsening symptoms. The study may help MS providers learn about possible effects from a therapy change. The study may give health care providers and patients new information to help make better therapy choices in the future.
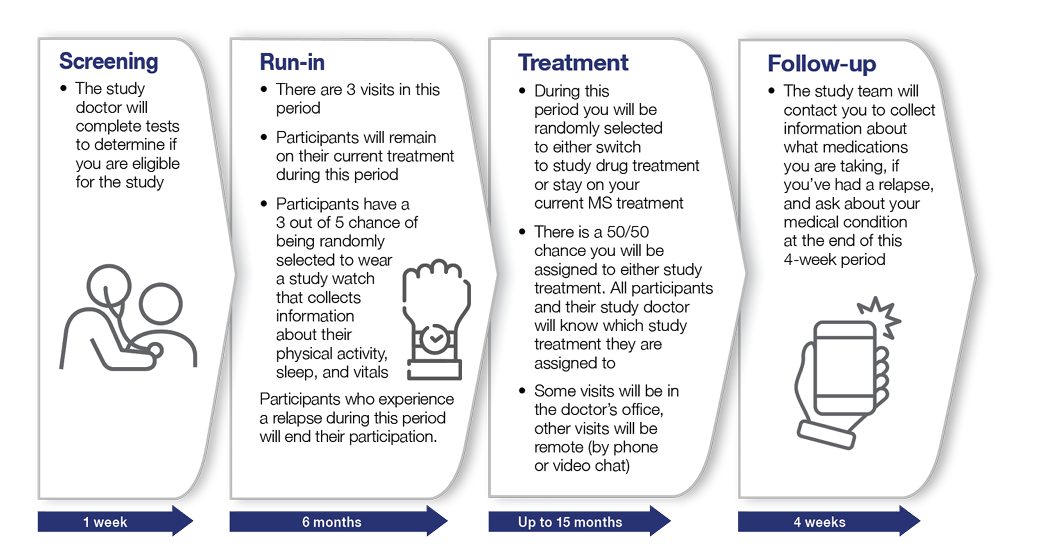
There are 4 periods of the study: the screening period, the run-in period, the treatment period, and the follow-up period. The figure below shows what will happen during each period.